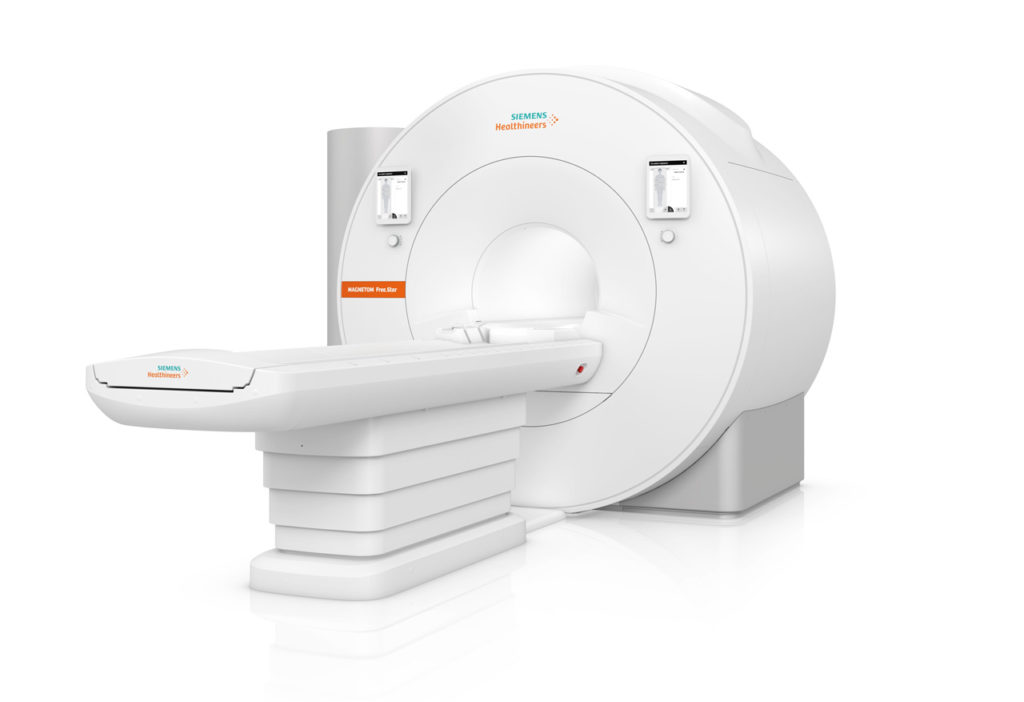
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the MAGNETOM Free.Star, a cost-effective whole-body magnetic resonance (MR) scanner designed to help improve patient access to MRI. The second scanner on the High-V MR platform following the MAGNETOM Free.Max, the MAGNETOM Free.Star has a 60 cm patient bore and combines a 0.55 Tesla (0.55T) field strength with deep learning technologies and advanced image processing.
The MAGNETOM Free.Star is the company’s most affordable MR scanner and at 3.3 tons and less than 80 inches high, its smallest, most lightweight whole-body MRI system ever. It requires less than 1 liter of liquid helium and no quench pipe, contributing to reduced infrastructure and lifecycle costs. The scanner’s reduced energy consumption contributes to a reduction in total lifecycle costs of more than 30 percent compared to conventional scanners. Deep Resolve algorithms perform targeted denoising and employ deep learning to deliver sharp, high-resolution images, elevating image quality to a level previously achievable only using MR scanners with much higher field strengths. The myExam Companion workflow solution leverages artificial intelligence to help the user conduct a more efficient patient examination.
“Siemens Healthineers believes that patients everywhere deserve access to magnetic resonance imaging and its unique benefits,” said Jane Kilkenny, Vice President of the
Magnetic Resonance business at Siemens Healthineers North America. “The MAGNETOM Free.Star is further proof of our steadfast commitment to providing customers with MRI scanners that are more cost-effective, more easily operable, and more easily sited for installation at a wide variety of healthcare institutions across the United States.”
For more information, see siemens-healthineers.us/magnetom-free-star.








