New SuperSonic MACH 20 ultrasound system designed to optimize costs while improving outcomes
Magazine
Philips Integrates 3D Ultrasound with Innovative Software for Breakthrough in Surveillance Of Abdominal Aortic Aneurysms
Jan 27, 2021
Royal Philips, a global leader in health technology, has introduced the Philips Abdominal Aortic Aneurysm (AAA) Model, providing physicians a more patient-friendly solution compared to the current standard of care for managing AAA patients.
Probo Medical Acquires Mount International United Services
Jan 26, 2021
Probo Medical, a global provider of medical imaging equipment, parts, repair and service, has acquired Mount International United Services Ltd (MIUS). Terms of the transaction were not disclosed.
In Memoriam | Anne Granum
Jan 25, 2021
“The HTM community lost another wonderful human being,” the LinkedIn post read.
Canon Medical’s AI-Powered, Premium Large Bore CT Receives FDA Clearance
Jan 22, 2021
Canon Medical Systems USA, Inc. has received FDA clearance for the Aquilion Exceed LB CT system, giving clinicians the opportunity to see more during radiation therapy planning for accuracy, precision and speed.
FDA Clears MULTIX Impact C Ceiling-Mounted DR System
Jan 21, 2021
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the MULTIX Impact C ceiling-mounted digital radiography (DR) system as well as the MULTIX Impact VA20, a new version of the established floor-mounted parent DR system.
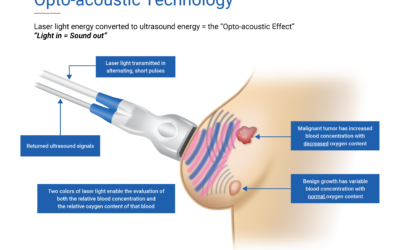
FDA Approves Seno Medical’s Breast Cancer Diagnostic Technology
Jan 21, 2021
The Center for Devices and Radiological Health (CDRH) of the U.S. Food & Drug Administration (FDA) has granted Texas-based Seno Medical Instruments Inc.
Philips to Acquire Capsule Technologies Inc.
Jan 19, 2021
Royal Philips has signed an agreement to acquire Capsule Technologies Inc., a provider of medical device integration and data technologies for hospitals and healthcare organizations.
HealthMyne Appoints National Oncology Leader to Board of Directors
Jan 19, 2021
HealthMyne, a pioneer in applied radiomics, has announced the appointment of Mimi Huizinga, MD, MPH, FACP, to its board of directors.
MITA Applauds CMS for Creation of Medicare Coverage Pathway for Innovative Medical Technologies
Jan 14, 2021
The Medical Imaging & Technology Alliance (MITA), the leading organization and collective voice of medical imaging equipment, radiopharmaceutical, contrast media, and focused ultrasound device manufacturers, today applauded the Centers for Medicare & Medicaid Services (CMS) for its recently issued final rule concerning the Medicare Coverage of Innovative Technology (MCIT) program.