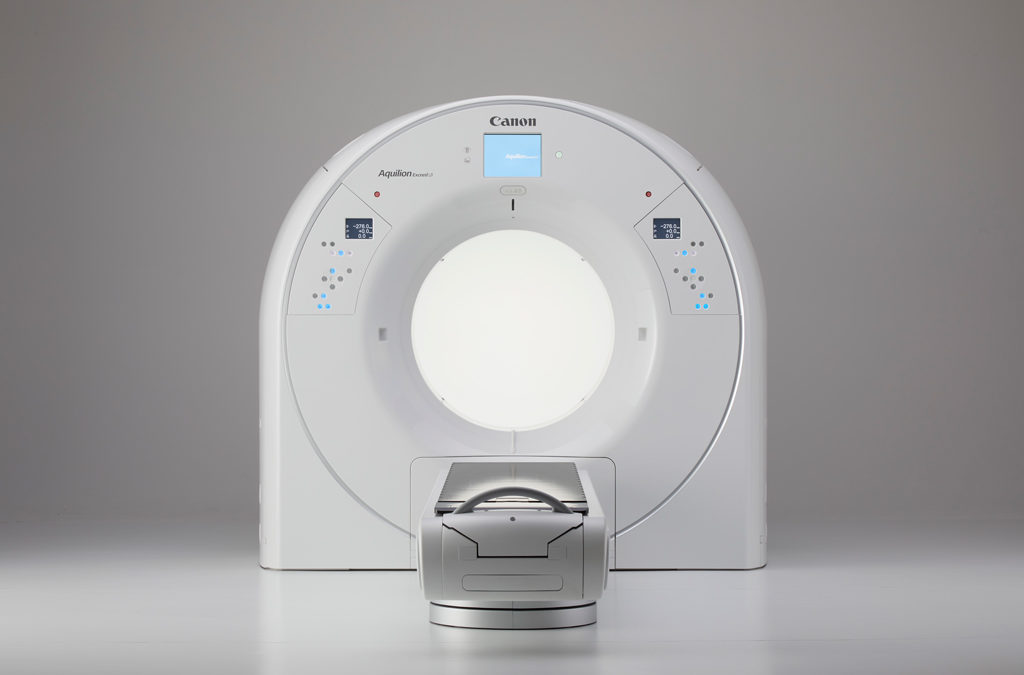
An estimated 1.8 million new cancer cases will be diagnosed in the U.S. this year alone, according to the American Cancer Society. With a disease so prevalent, treatments like radiation therapy are critical, and while planning, clinicians require accuracy, precision and speed. Giving clinicians the opportunity to see more during radiation therapy planning, Canon Medical Systems USA, Inc. is introducing the Aquilion Exceed LB CT system (pending 510(k) clearance) during this year’s virtual American Society for Radiation Oncology (ASTRO) Annual Meeting.
The Aquilion Exceed LB can help Radiation Oncologists with fast and efficient radiation oncology workflows without compromising on patient position, image quality or reproducibility. Features include:
- Accuracy in even the most complex simulations through industry-leading capabilities, like the largest bore opening (90 cm), edge-to-edge extended Field-of-View (90 cm) reconstruction and widest detector coverage (4 cm).
- Better contouring using Artificial Intelligence (AI) with sharp, clear and distinct images from Canon Medical’s Advanced intelligent Clear-IQ Engine (AiCE) Deep Learning Reconstruction (DLR) technology.
- Expanded capabilities – because of the Aquilion Exceed LB’s suite of premium CT capabilities, the system is positioned for shared services to enable high-quality treatment follow-up and interventional procedures.
“Accurate simulation across even the most challenging treatment plans is imperative during radiation therapy planning,” said Erin Angel, managing director, CT Business Unit, Canon Medical Systems USA Inc. “At Canon Medical, we deliver solutions that meet providers’ needs but also push the boundaries of traditional simulation. With the Aquilion Exceed LB, we brought deep learning reconstruction to the CT simulation space. This will revolutionize care for cancer patients and give the radiation oncology team the confidence and precision they need for accurate planning across patients.”
Learn more about Canon Medical’s new Exceed LB CT system by visiting the virtual booth during ASTRO, October 23-29, 2020.