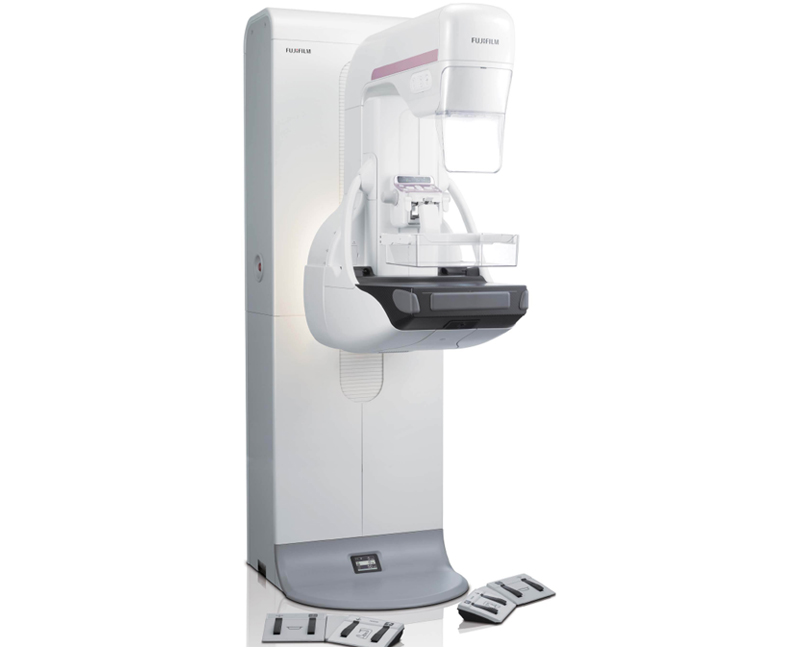
Fujifilm Medical Systems U.S.A. Inc. earlier this year announced that its Digital Breast Tomosynthesis (DBT), as an optional software upgrade for its ASPIRE Cristalle digital mammography system, has received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA). Known as AMULET Innovality outside of the United States, Fujifilm’s optional DBT upgrade has been widely available in Europe, Asia and Latin America since May 2013. The ASPIRE Cristalle FFDM system with DBT combines Fujifilm’s state-of-the-art hexagonal close pattern (HCP) detector design, advanced image processing and image acquisition workflow to optimize patient dose while maximizing image quality.