Company expands breast specimen imaging portfolio and leadership in breast cancer diagnosis and care
 Hologic, Inc. (Nasdaq: HOLX) today announced global commercial availability of the Trident® HD specimen radiography system, a next-generation solution that delivers enhanced image quality, improved workflow and instant sample verification during breast-conserving surgeries and stereotactic breast biopsies.1
Hologic, Inc. (Nasdaq: HOLX) today announced global commercial availability of the Trident® HD specimen radiography system, a next-generation solution that delivers enhanced image quality, improved workflow and instant sample verification during breast-conserving surgeries and stereotactic breast biopsies.1
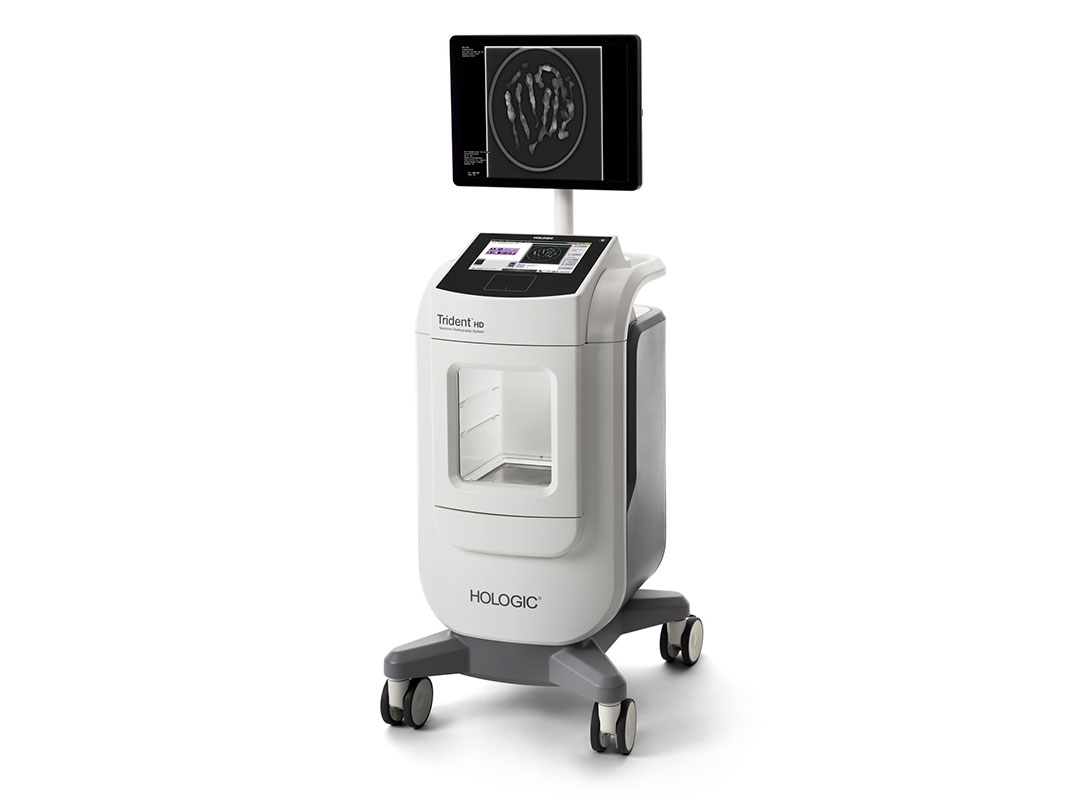
The Trident products are the only specimen radiographs on the market to use amorphous selenium direct capture imaging – the same detector technology used in Hologic’s 3Dimensions™ mammography system – to generate crisp, clear, high-resolution images. The new Trident HD system, which recently received FDA clearance in the US and a CE Mark in Europe, also features a bigger detector that allows for complete imaging of larger breast surgical specimens, along with a wide range of surgical and biopsy samples.2
“The Trident HD system is a breakthrough solution that delivers the superior image quality clinicians have come to expect from Hologic products, helping to streamline workflows and reduce recalls while decreasing procedure times,” said Pete Valenti, Hologic’s Division President, Breast and Skeletal Health Solutions. “We are committed to identifying and addressing the challenges of our customers and their patients at every step of the breast health journey, and our expanding product portfolio is evidence of that commitment.”
The Trident HD system eliminates the need for clinicians to transport specimens for imaging and features an ergonomic design that is 37 percent smaller than the original Trident system, making it easy to maneuver in a crowded operating or procedure room. Prior mammography or biopsy images can be displayed on the same Trident HD high-resolution monitor to speed comparison and analysis, resulting in reduced procedure time and improved workflow.1 Additionally, an intuitive touchscreen interface and wireless integration supports advanced image sharing and seamless transfer of patient records to the facility’s picture archiving and communication system (PACS).
The market leader in mammography, Hologic has expanded its product suite significantly in recent months through insight-driven, innovative product launches and strategic acquisitions to address the continuum of breast health care. In addition to the Trident HD system, Hologic recently added the LOCalizer™ wireless radio frequency identification (RFID) breast lesion surgical guidance system, which is available in the U.S. and Europe. The Company also offers the BioZorb® 3D bioabsorbable marker, an implantable three-dimensional marker that enables more targeted radiation therapy and helps clinicians overcome challenges in breast-conserving surgery or lumpectomy. BioZorb is currently available in the U.S. only. This expanded portfolio enables Hologic to play a larger role in breast-conserving surgery and further strengthen its offerings to radiologists, pathologists and breast surgeons.
For more information about the Trident HD system, please visit here.