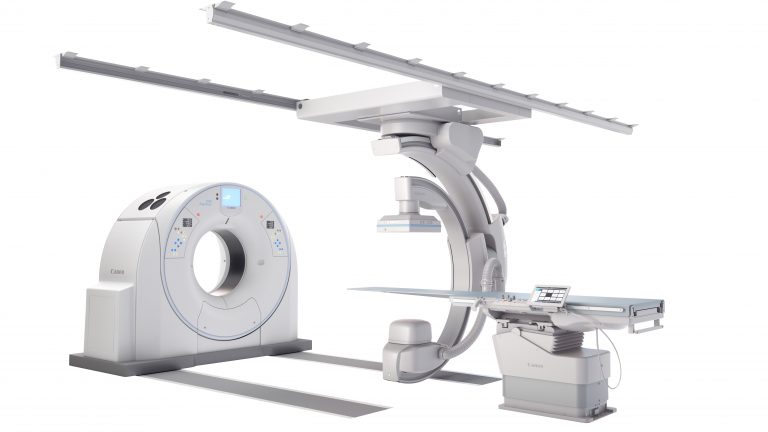
At this year’s Society of Interventional Radiology (SIR) 2019, Canon Medical Systems USA, Inc. is showcasing its new, advanced angiography configuration, the Alphenix™ 4D CT, which features its Alphenix Sky + C-arm and Hybrid Catheterization Tilt/Cradle Table for interventional procedures with its premium AquilionT™ ONE / GENESIS Edition CT system. The new pairing offers a fully integrated interventional imaging system to perform a wide variety of procedures, allowing clinicians to efficiently plan, treat and verify in a single clinical setting. The flexible hybrid system enables streamlined workflow and outstanding range of patient access and coverage.
This 4D CT configuration is part of the new family of Alphenix angiography systems which delivers images with clarity and precision. The next-generation family of systems combines industry-leading dose optimization technologies, enhanced workflow and innovative features to help clinicians provide patients with safe and fast treatment. The Alphenix 4D CT was recently launched during RSNA 2018 and has been installed in the U.S. at West Virginia University Heart and Vascular Institute.
“With the hybrid catheterization tilt/cradle table and workflow enhancements permitted by the new Alphenix Workstation, clinicians can attain unparalleled flexibility and discover new opportunities for novel procedures through intervention,” said Casey Waldo, director, Vascular Business Unit, Canon Medical Systems USA, Inc. “The new configuration enables physicians to expand their capabilities with 4D CT – particularly in fast-growing markets such as interventional oncology for minimally invasive tumor treatment, where the system enables physicians to image changes in the tumor and adjust treatment, then use the CT system to evaluate the results.”
Canon Medical Systems will showcase its Alphenix 4D CT system at this year’s Society of Interventional Radiology (SIR) 2019 and during a SIR Lunch Symposium on Sunday, March 24, 2019, 12:00 p.m. – 1:00 p.m. at the Austin Convention Center, Room 6A, 500 E. Cesar Chavez St., Austin, TX, where attendees will have the opportunity to get an exclusive insight into the unique use of Alphenix 4D CT in Interventional Radiology/Oncology from renowned experts in the field.
The Alphenix 4D CT is part of Canon Medical’s suite of Collaborative Imaging tools which puts integrated imaging intelligence at the center of a patient’s journey. The initiative fuses multiple diagnostic imaging modalities with leading clinical applications to deliver holistic, optimized patient information to health care providers at the point of care.







