COVER STORY
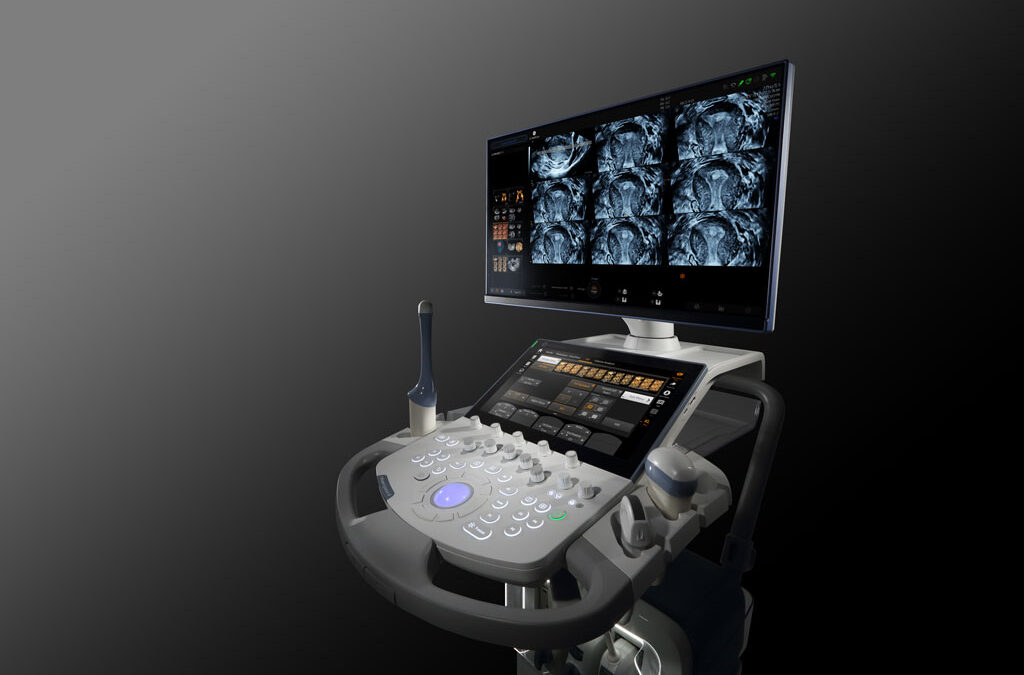
GE HealthCare Aims to Advance Women’s Health Imaging
GE HealthCare recently launched the Voluson Signature 20 and 18 ultrasound systems, which comprehensively integrate artificial intelligence (AI), advanced tools and an ergonomic design to speed exam time for clinicians while delivering a clearer picture of various...

FDA Releases Two Companion Reports on Medical Device Safety and Innovation
Today, the U.S. Food and Drug Administration’s (FDA) Center for Devices and Radiological Health Center (CDRH) is releasing two reports on medical device safety and innovation – the core pillars that help protect and promote public health for all. The “CDRH 2024 Safety...

Tri-Imaging, RTI Group Work Together
In a LinkedIn post, Tri-Imaging states, “We are honored to be the first ISO in North America to have the opportunity to try RTI’s new Mako X-Ray Testing Meter. The Mako meter is the most accurate and efficient testing meter that covers the broadest application range...
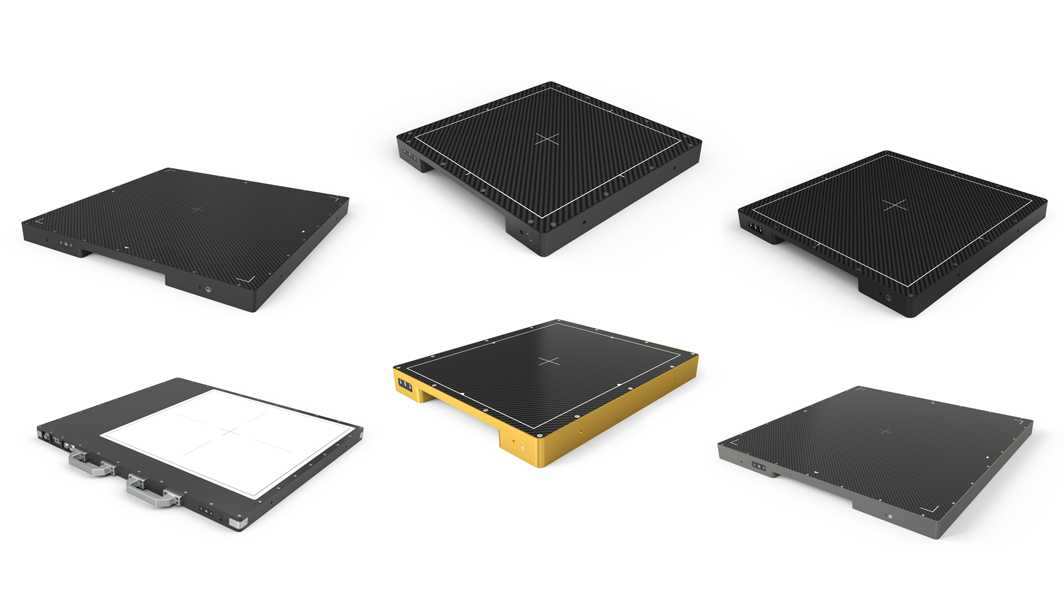
Detection Technology announces global availability of TFT flat panel detectors
Detection Technology, a global leader in X-ray detector solutions, announces the global availability of a comprehensive range of TFT (thin-film transistor) flat panel detectors. The portfolio includes IGZO (indium gallium zinc oxide) and a-Si (amorphous silicon)...

Bayer, Google Cloud Accelerate Development of AI-Powered Applications for Imaging
Bayer and Google Cloud have announced a collaboration on the development of artificial intelligence (AI) solutions to support radiologists and ultimately better serve patients. As part of the collaboration, Bayer will further develop its innovation platform to...

AHRA Announces ‘Living Our Legacy’ Campaign
For over 50 years, the Association for Medical Imaging Management (AHRA) has been the premier professional organization that represents the voices of more than 5,000 medical imaging leaders in over 2,500 health care facilities. AHRA, aligned with its philanthropic arm...
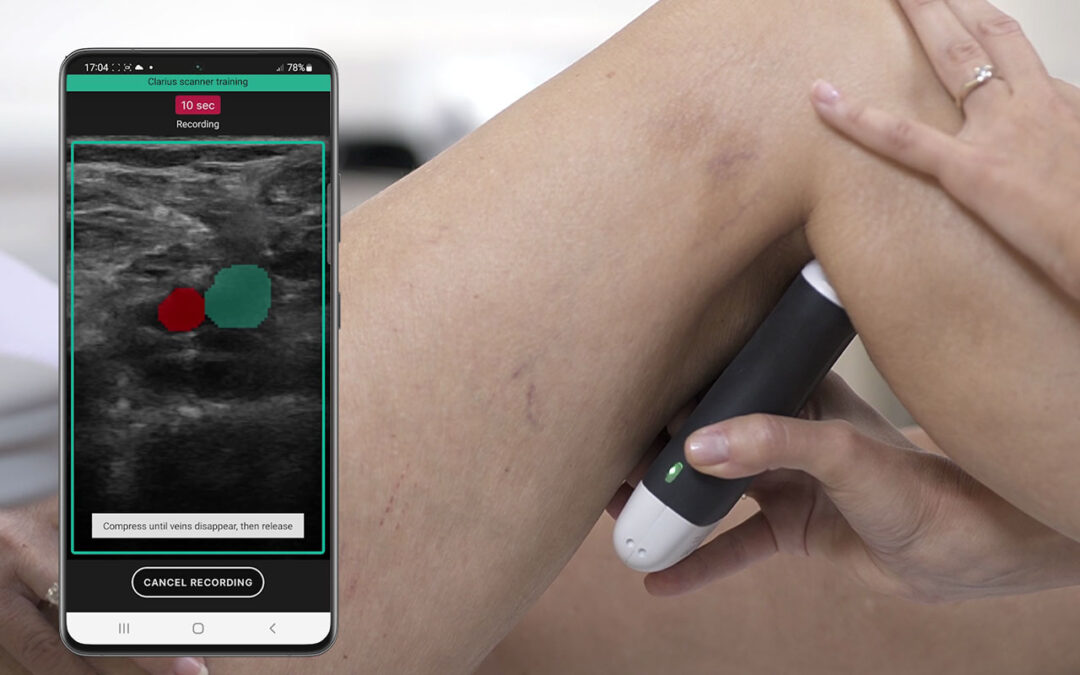
AI-Guided Ultrasound System Enables Rapid DVT Assessment
Clarius Mobile Health, a provider of high-definition handheld ultrasound systems, and ThinkSono, a company specializing in ultrasound artificial intelligence (AI) guidance solutions, are introducing a new AI-guided ultrasound system in Europe, which will improve the...
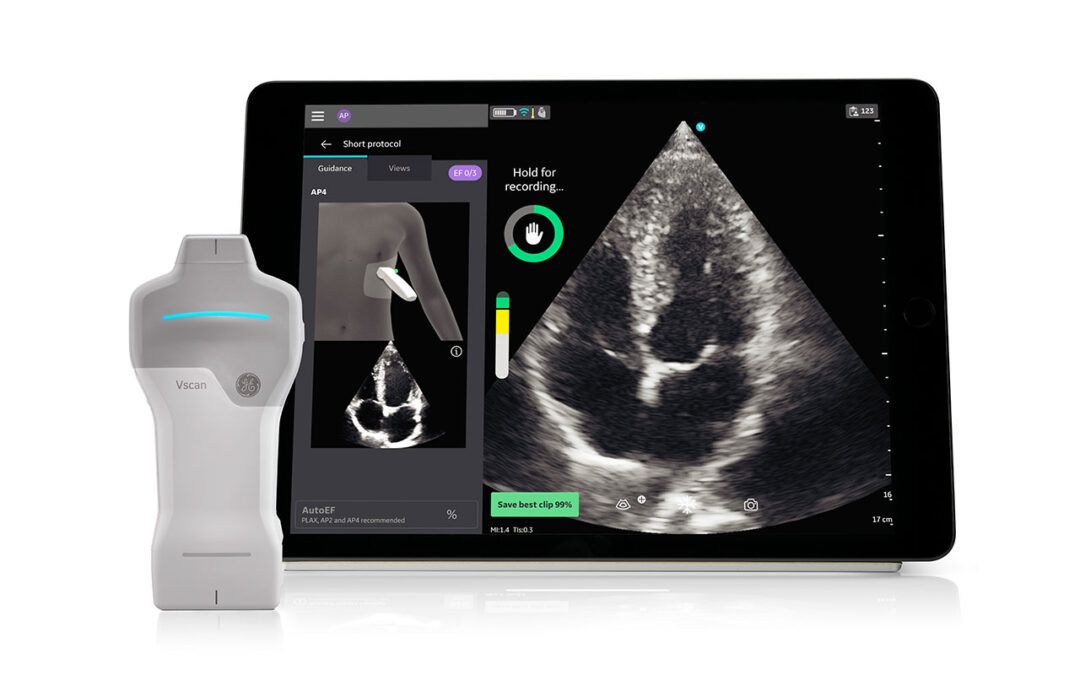
GE HealthCare Introduces Caption AI on Vscan Air SL Wireless Handheld Ultrasound System
GE HealthCare recently launched Caption AI artificial intelligence (AI)-driven software for rapid cardiac assessments at the point of care on Vscan Air SL. Now, with Caption AI technology, clinicians using Vscan Air SL handheld ultrasound will have access to...
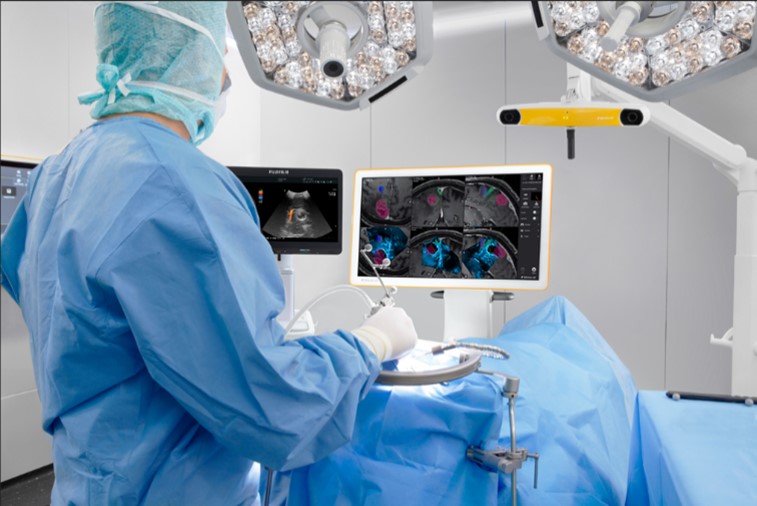
Brainlab, Fujifilm Offer Advanced Neurosurgery Capabilities
FUJIFILM Healthcare Americas Corporation and Brainlab recently announced that Brainlab will be the exclusive U.S. distributor of ARIETTA Precision Ultrasound for neurosurgery applications to be utilized with Brainlab’s surgical navigation systems. ARIETTA Precision,...

MAMMOMAT B.brilliant Mammography System Earns 510(k) Clearance
Siemens Healthineers recently announced the Food and Drug Administration 510(k) clearance of the MAMMOMAT B.brilliant – the first completely redesigned mammography platform in more than 10 years. The clearance includes elements involving full-field digital...




