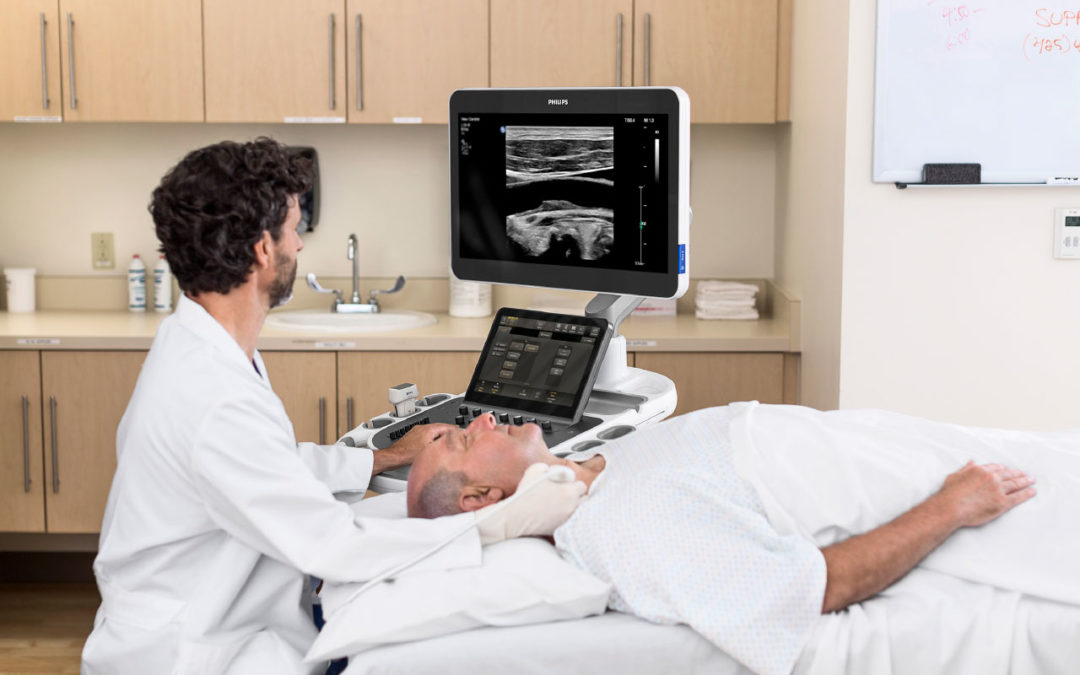
The EPIQ Elite ultrasound system is a new premium ultrasound that combines the latest advances in transducer innovation and enhanced performance to improve clinical confidence and the patient experience. EPIQ Elite offers a range of diagnostic ultrasound solutions tailored to the needs of specific medical specialties, including Philips’ first solution for vascular assessment and diagnosis. The Philips Ultimate Ultrasound Solution for Vascular Assessment combines 3D and 4D imaging, a breakthrough simplified workflow and complimentary clinical tools to effectively assess and monitor vascular disease.
The EPIQ Elite ultrasound system is a new premium ultrasound that combines the latest advances in transducer innovation and enhanced performance to improve clinical confidence and the patient experience. EPIQ Elite offers a range of diagnostic ultrasound solutions tailored to the needs of specific medical specialties, including Philips’ first solution for vascular assessment and diagnosis. The Philips Ultimate Ultrasound Solution for Vascular Assessment combines 3D and 4D imaging, a breakthrough simplified workflow and complimentary clinical tools to effectively assess and monitor vascular disease.