Royal Philips is showcasing innovations at EuroPCR (May 17-20, Paris, France) that can enable interventionists to perform ultra-low contrast percutaneous coronary intervention (ULC-PCI) procedures with greater confidence and clarity. Philips’ ULC-PCI solutions co-register instantaneous blood flow measurements and/or intravascular ultrasound (IVUS) images onto real-time fluoroscopy to help interventionists diagnose, decide, guide, treat and confirm the success of PCI, with the potential to limit the use of iodinated contrast media.
Seamlessly integrating into Philips’ Image Guided Therapy System – Azurion – the company’s unique ULC-PCI solutions provide physicians with tools to help reduce the use of contrast media throughout PCI procedures.
The ability to perform PCI procedures using a very small amount of contrast media enables PCI to be offered to more patient groups, notably patients presenting with both coronary artery disease (CAD) and chronic kidney disease (CKD), who are at high risk of suffering contrast-induced nephropathy (CIN) [1] – a life-threatening form of hospital-acquired acute kidney injury (AKI) caused by contrast media toxicity. A 2020 study in the USA concluded that AKI after a PCI procedure resulted in an average increase in length of hospital stay of 3.6 days and an additional healthcare cost of 9,448 USD per patient [2].
“Innovation in catheter-based interventions to treat narrowed heart arteries – so-called percutaneous coronary interventions – continuous to contribute to improving the quality of life and prognosis for millions of patients around the world,” said Javier Escaned, MD, PhD, Head of the Interventional Cardiology Section at Hospital Clinico San Carlos, Madrid, Spain. “As a result, more complex patients can now undergo PCI, including those with advanced age and frailty, chronic renal failure, and associated heart conditions. In many of these patients, where the injection of radiological contrast used to guide the PCI can have deleterious effects, technologies developed by Philips that enable physicians to dramatically decrease contrast administration during the procedure is contributing to both the safety and quality of PCI.”
Dynamic Coronary Roadmap
During a conventional PCI procedure, contrast media is injected into the patient’s coronary arteries to acquire an angiogram, with additional fluoroscopy used during the procedure to help interventionists navigate their guide wires and catheters. To maintain visibility of the arteries, this guidance typically requires repeated contrast media injections, increasing the toxic load on the patient’s kidneys. Philips’ Dynamic Coronary Roadmap software removes the need for additional contrast media injection by overlaying the preoperative angiogram onto real-time motion-compensated 2D fluoroscopic imaging to provide interventionists with continuous visual feedback on the positioning of guide wires and catheters. In many cases, no additional contrast media injection is required for wire navigation.
While Philips’ Dynamic Coronary Roadmap software helps interventionists navigate guide wires and catheters to the site of a lesion, the company’s IntraSight Series 7 precision guidance system streamlines lesion assessment, simplifies vessel sizing, and enables precise therapy delivery.
iFR Co-registration
As an alternative or adjunct to IVUS co-registration, spatially accurate instantaneous wave-free ratio (iFR) pullback measurements can be co-registered onto the angiogram, adding valuable physiological data to the anatomical imaging. Unlike fractional flow reserve (FFR) measurements, iFR measurements do not require the use of hyperemic drug injection and can be used to assess both the degree and length of vessel stenosis and the effectiveness of therapy using a simple pressure wire pullback technique.
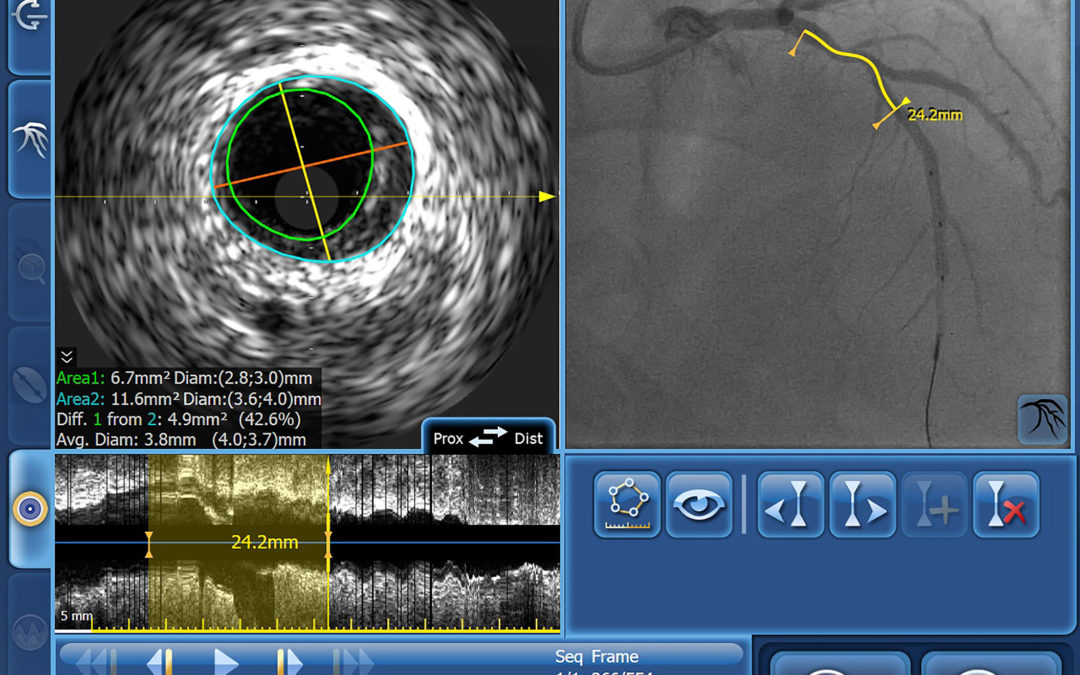
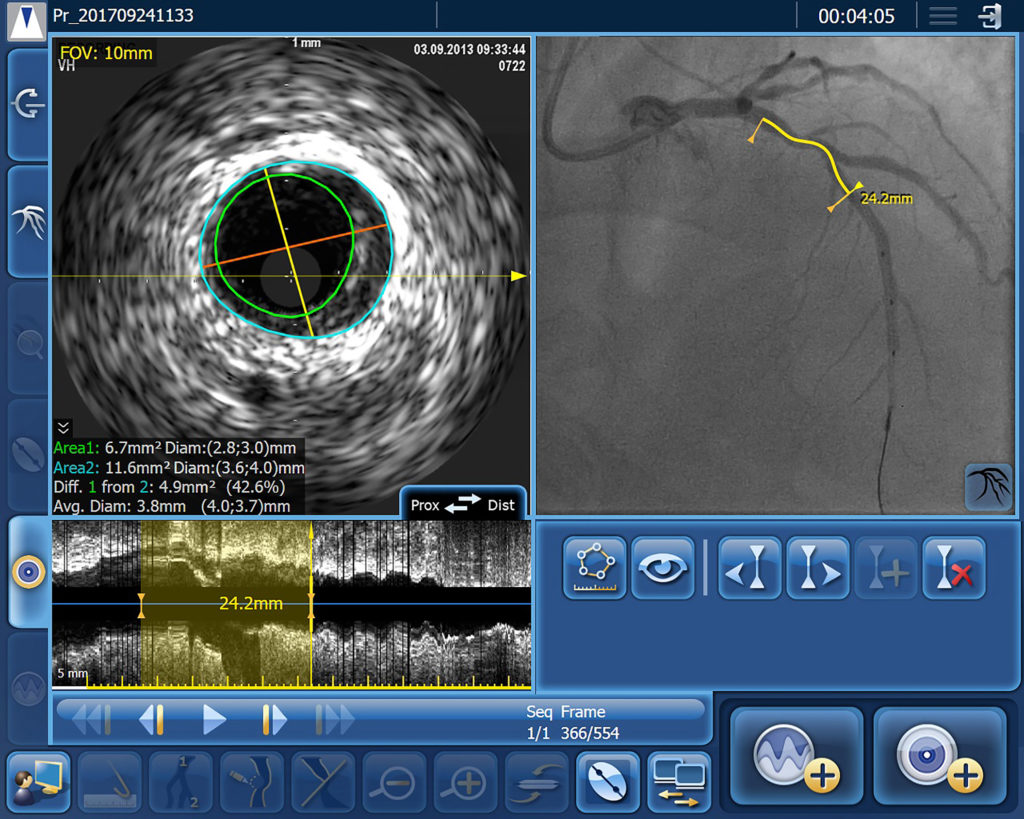
IVUS Co-registration
IntraSight Series 7’s IVUS co-registration facility merges real-time intravenous ultrasound and angiogram images, with information on the precise location of the ultrasound images derived during manual pull-back of the ultrasound catheter under continuous fluoroscopy. As a result, interventionists can simultaneously view reconstructed cross-sectional ultrasound images of the vessel lumen, including their precise position on the angiogram. This level of precision significantly reduces the risk of a ‘geographic miss’, which has been estimated to occur in over 60% of PCIs [3]. IntraSight Series 7’s Angio+ quantitative coronary analysis software automatically calculates lumen dimensions and stenosis in real time, helping accurate assessment of the required stent size.
Tri-registration
IntraSight Series 7’s Tri-registration function aids stent selection by co-registering IVUS and iFR information with the angiogram to help choose a stent that optimally supports meeting a procedure’s objectives. Coupled with IntraSight Series 7’s enhanced live stent visualization capabilities, which help to immediately verify correct stent positioning and deployment, the ability to enhance stent choice and size means more right-first-time procedures and better patient outcomes.
Ultra-Low Contrast PCI at EuroPCR
Professor Javier Escaned, together with other key thought leaders in the field of UCL-PCI, will discuss how to decrease operator dependence on vessel opacification during PCI in a Philips-sponsored symposium at EuroPCR 2022 on Tuesday, May 17, from 17:15 to 18:15 (CET). For more information and registration, click here.
[1] Dangas G, et al. Contrast-Induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. AJC 2005. https://doi.org/10.1016/j.amjcard.2004.08.056.
[2] Amin P, et al. Incremental cost of Acute Kidney Injury after Percutaneous Coronary Intervention in the United States. AM J Cardiol. 2020 Jan1;125(1):29-331.
[3] Costa et al. Impact of Stent Deployment Procedural Factors on Long-term Effectiveness and Safety of Sirolimus-Eluting Stents (Final results of the Multicenter Prospective STLLR Trial), Am J Cardiol 2008 Jun 15; 101(12):1704-11.