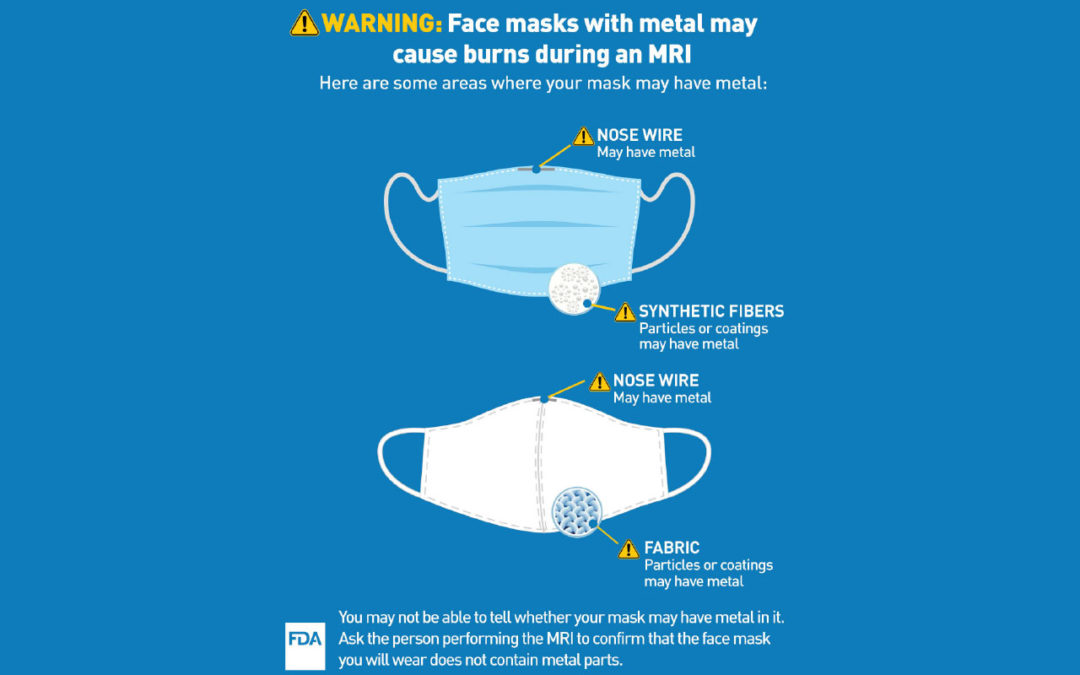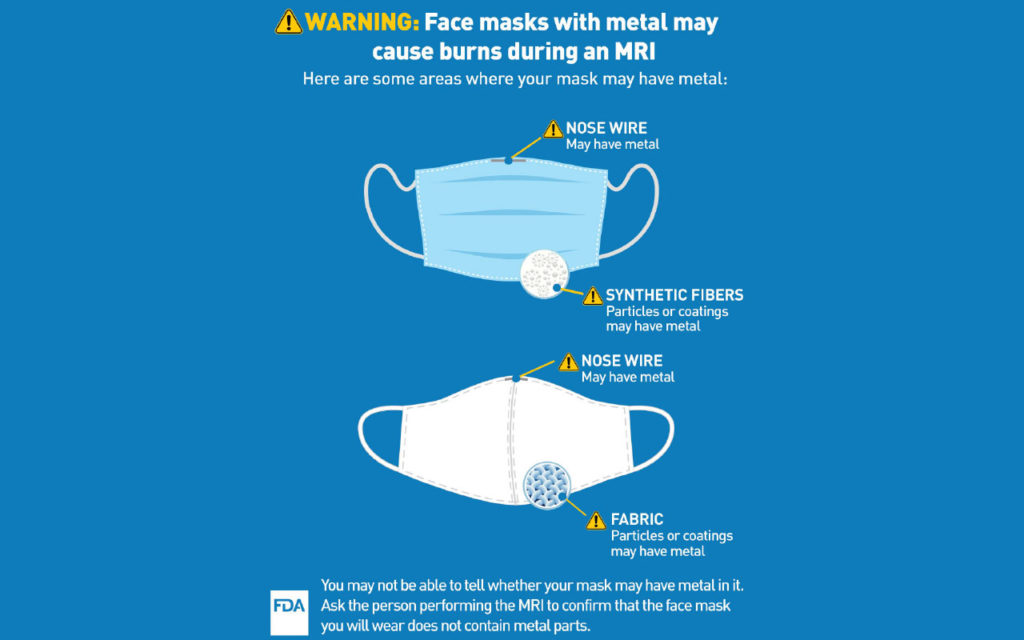
The U.S. Food and Drug Administration (FDA) is informing patients and health care providers that patients may be injured if they wear face masks (such as surgical or non-surgical masks and respirators) with metal parts and coatings during a magnetic resonance imaging (MRI) exam. Metal parts, like nose pieces sometimes called nose clips or wires, nanoparticles (ultrafine particles), or antimicrobial coating that may contain metal (such as silver or copper), may become hot and burn the patient during an MRI. The FDA recommends patients wear face masks with no metal during MRIs.
When it is appropriate for a patient to wear a face mask during an MRI exam, such as during the COVID-19 public health emergency, ensure the face mask contains no metal. Some face masks include flexible parts, nose pieces, headband staples, nanoparticles, or antimicrobial coating that may contain metal. If the absence of metal cannot be confirmed and it is determined to be appropriate for the patient to wear a face mask, an alternative face mask where the absence of metal can be confirmed should be used. Health care providers who perform MRI exams are encouraged to provide face masks without metal to patients who will undergo an MRI.
Continue to screen all patients for MRI safety, including looking for metallic objects, prior to MRI examinations.
If a patient experiences an adverse event, such as a burn, while wearing a face mask during an MRI, you are encouraged to report the event to the FDA. Your report, along with data from other sources, can provide information that helps improve patient safety.
For more information, visit tinyurl.com/MRImasks.