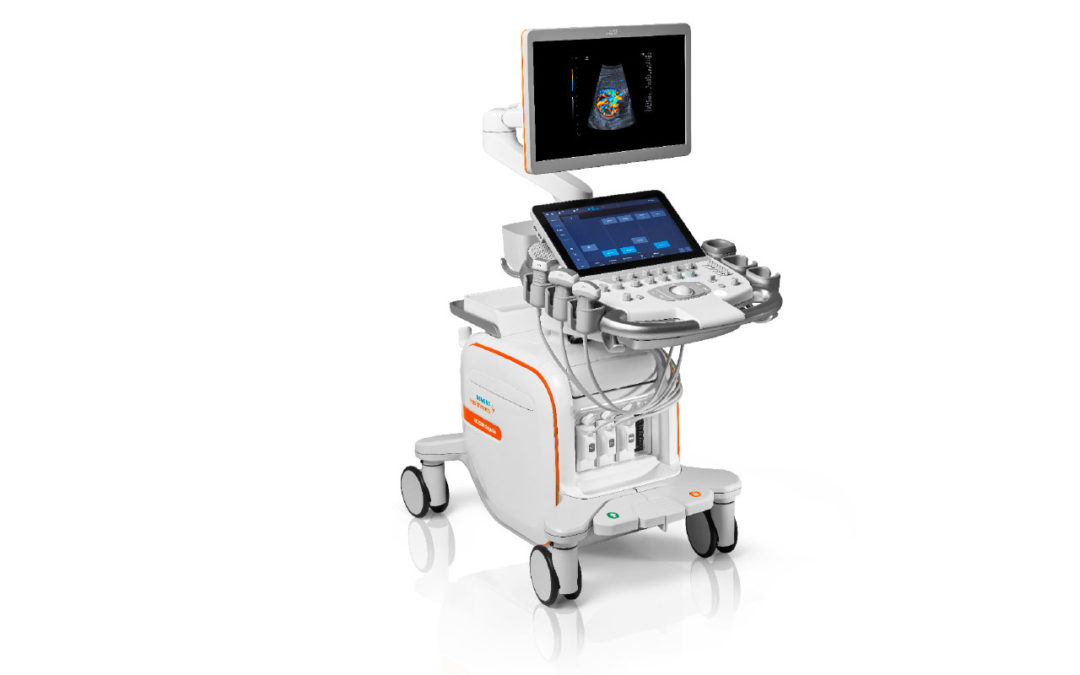
With patient-centric technology and applications at the core of its DNA, the ACUSON Sequoia ultrasound system with BioAcoustic technology can adapt to patient’s unique characteristics. Built from the ground up with input from users around the world, the ACUSON Sequoia system was created with users and patients in mind. An unmatched list of advanced applications offerings allows clinicians to personalize ultrasound to a patient’s specific needs. Powerful AI-enabled tools and user-centric interfaces improve workflow efficiency allowing clinicians to focus more on their patients.