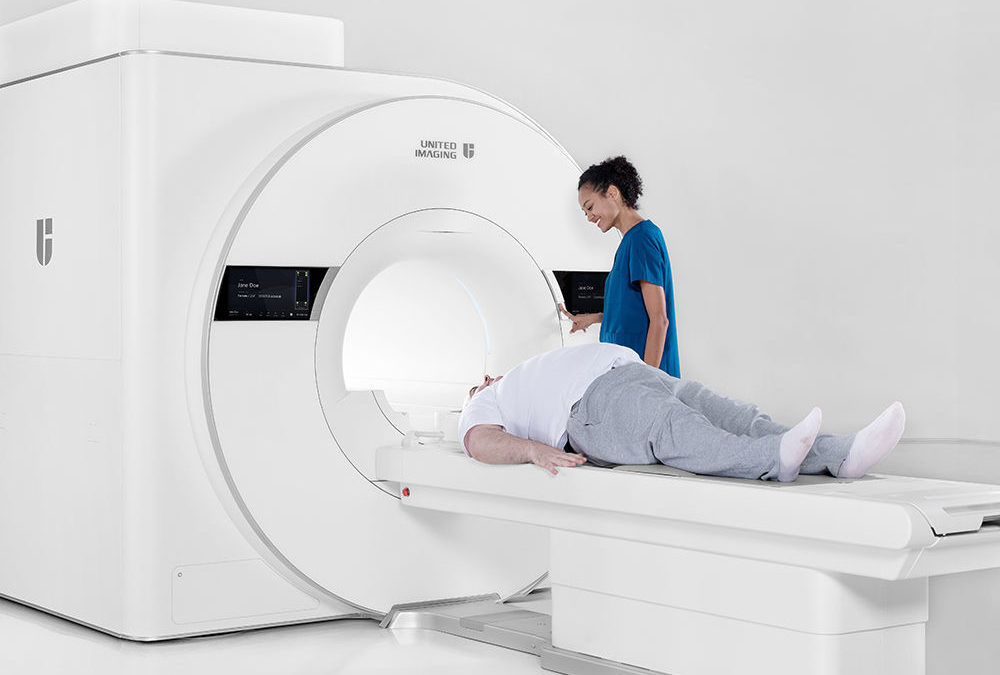
United Imaging announced U.S. Food & Drug Administration (FDA) clearance of the uMR OMEGA magnetic resonance imaging (MRI) scanner. The uMR OMEGA offers the world’s first ultra-wide 75-cm bore.[1] With the widest bore in the market at any field strength, uMR OMEGA provides a unique, patient-centric experience designed to accommodate a wide range of patients.
“The uMR OMEGA is a significant breakthrough. With this new scanner, United Imaging addresses the needs of health care providers to comfortably accommodate more patients in their community, including much of the 40 percent of the U.S. population suffering from obesity[2] and the 2.5 percent of the population suffering from claustrophobia,[3]” said Jeffrey Bundy, Ph.D., chief executive officer at UIH Solutions. “The uMR OMEGA represents a critical advance for patients who will now have access to an enhanced patient experience and a more accommodating MRI scanning environment. This FDA clearance is another huge milestone for our company, as we continually work to set standards in all modalities and make these state-of-the-art capabilities accessible to more communities in the United States and abroad. We are proud to add uMR OMEGA to our already expansive portfolio of medical imaging devices.”
With an industry-leading 75-cm bore and 680-pound table capacity,[1] uMR OMEGA addresses evolving demographics in the U.S. community, not only improving patient comfort for all but creating, for the first time, an MRI for bariatric patients. The scanner adeptly serves pediatric and geriatric patients with faster scanning for both children and seniors who cannot stay still for long periods of time. Health care providers can offer new services, such as acute imaging in the emergency room with an ultra-fast 5-minute stroke protocol and cardiac imaging with a single breath-hold that significantly reduces the number of required scans.[4]
“At United Imaging, we are all driven by patient experience: focusing on it, understanding it, improving it,” said Abram Voorhees, vice president of MR. “We intimately understand the anxiety that individuals can experience while undergoing an MRI, as well as the physical limitations of MRI machines today that can make a potentially life-saving study inaccessible. uMR OMEGA was created to remove the obstacles that often limit access for patients. Today’s important announcement brings to light a critical need for our communities — and health care overall.”
Added CEO Bundy, “I’ve seen first-hand over the past decades how meaningful comfort is to patients in a bore, and it was important to us that we innovated in a way that removes barriers to care for more people.”
The U.S. is the first country in the world to receive uMR OMEGA. It received its FDA clearance in March and will be installed in United Imaging’s Houston showroom facility in the summer of 2020.
References
1. Data on file; https://www.ecri.org/, retrieved 1/27/2020
2. https://www.cdc.gov/, retrieved 1/23/2020
3. Data on file; Reduction of claustrophobia during magnetic resonance imaging: methods and design of the “CLAUSTRO” randomized controlled trial (2011), Enders et al retrieved 1/24/2020
4. Data on file, based on internal calculation of non-accelerated imaging vs uCS 2.0 and EasyScan, 1/28/2020