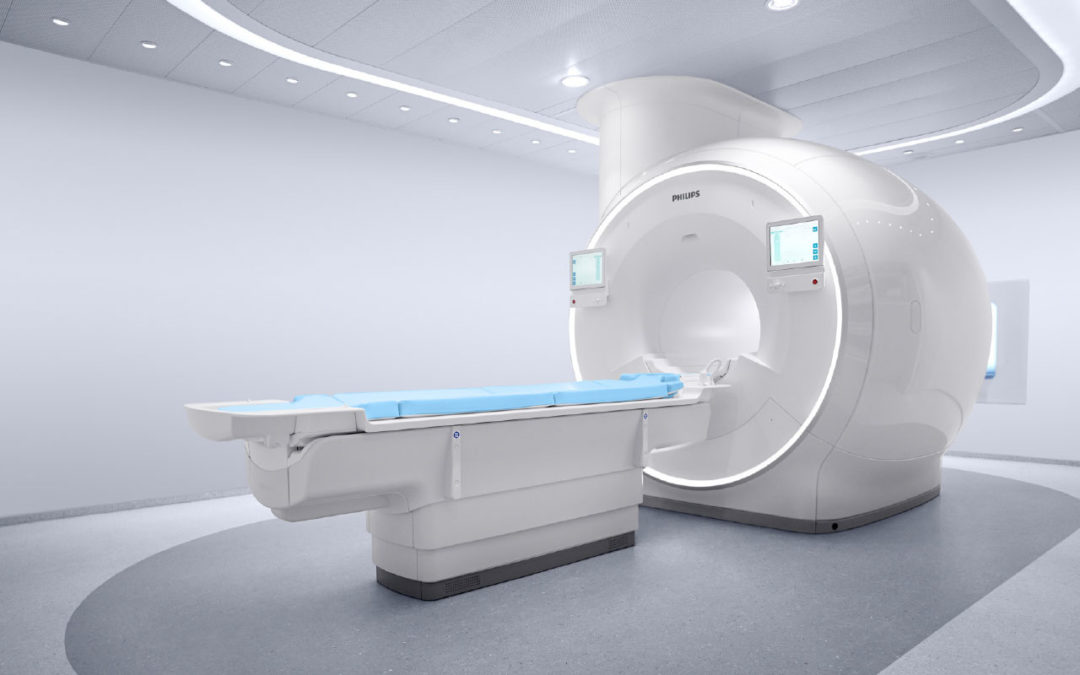
Incorporating Philips’ breakthrough BlueSeal fully sealed magnet, the Ingenia Ambition 1.5T is the world’s first and only commercially available MR system to enable helium-free operations[1], reducing the chance of potentially lengthy and costly disruptions, and virtually eliminating dependency on a commodity with an unpredictable supply. The Ingenia Ambition delivers high-quality images and performs MRI exams up to 50% faster with Compressed SENSE acceleration for all anatomies in both 2D- and 3D scanning. It also offers an immersive audio-visual experience to calm patients and guide them through MR exams.
1. The Ingenia Ambition 1.5T contains less than 0.5% of the helium of a conventional system and this is permanently sealed inside the device.